Published by Dr. Brandon Richland, MD
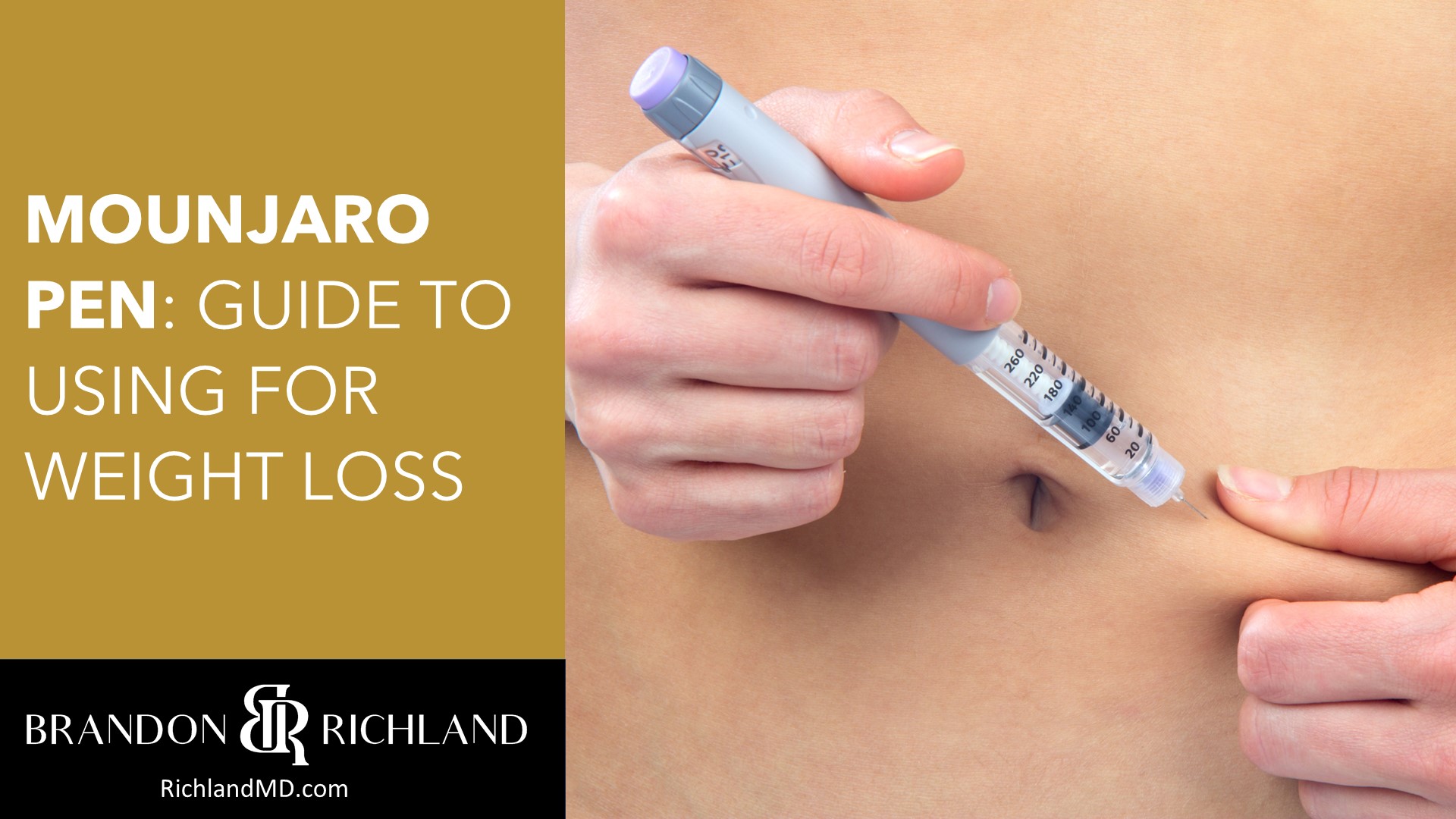
The Mounjaro single dose pen represents a step forward in the management of type 2 diabetes, offering a new option for those seeking better control over their blood glucose levels. As an injectable prescription medicine, Mounjaro, known generically as tirzepatide, is used in conjunction with diet and exercise to improve glycemic control in adults.
It operates by activating GIP and GLP-1 receptors, which play a role in regulating insulin secretion and blood sugar levels. Notably, while Mounjaro is intended for the treatment of type 2 diabetes, it is not suitable for those with type 1 diabetes.
Understanding how to use the Mounjaro pen properly is crucial for patients to get the maximum benefit from the medication. The pen is designed for ease of use, with a single-dose prefilled mechanism and clear instructions to guide patients through the process.
It’s meant for weekly subcutaneous injections, and patients can administer it at a site that is most comfortable for them, potentially enabling more consistent adherence to their treatment regimen. When considering Mounjaro, it’s important also to understand the dosing recommendations, potential side effects, and contraindications through discussions with healthcare providers.
Key Takeaways of Mounjaro Pen
- Mounjaro pen is a once-weekly dose injectable medication for improving blood sugar in type 2 diabetes.
- The pen is user-friendly, designed for self-administration without the need to mix or handle needles.
- Proper dosing and usage are essential for effectiveness and minimizing side effects.
Understanding Mounjaro
Mounjaro represents a significant advancement in the management of Type 2 diabetes.
What is Mounjaro?
Mounjaro is a brand name for the medication tirzepatide, used to manage Type 2 diabetes. It is available in a single-dose, pre-filled pen designed for ease of use. The medication works by mimicking two hormones that are essential for controlling blood sugar levels. With a unique mode of action, Mounjaro has become an important option for many individuals looking to maintain better sugar control.
- Administration: Once weekly via subcutaneous injection
- Dosage Forms: Available in multiple dosages to allow dose escalation
The Role of Tirzepatide
Tirzepatide, the active component in Mounjaro, plays a dual role in blood sugar regulation by mimicking the effects of incretin hormones GLP-1 and GIP. These hormones are naturally occurring and play pivotal roles in managing blood glucose. By harnessing their effects, tirzepatide.
- Lower Blood Sugar: Helps in reducing blood glucose levels post meals
- Weight Management: May assist in weight loss, a common comorbidity in Type 2 diabetes
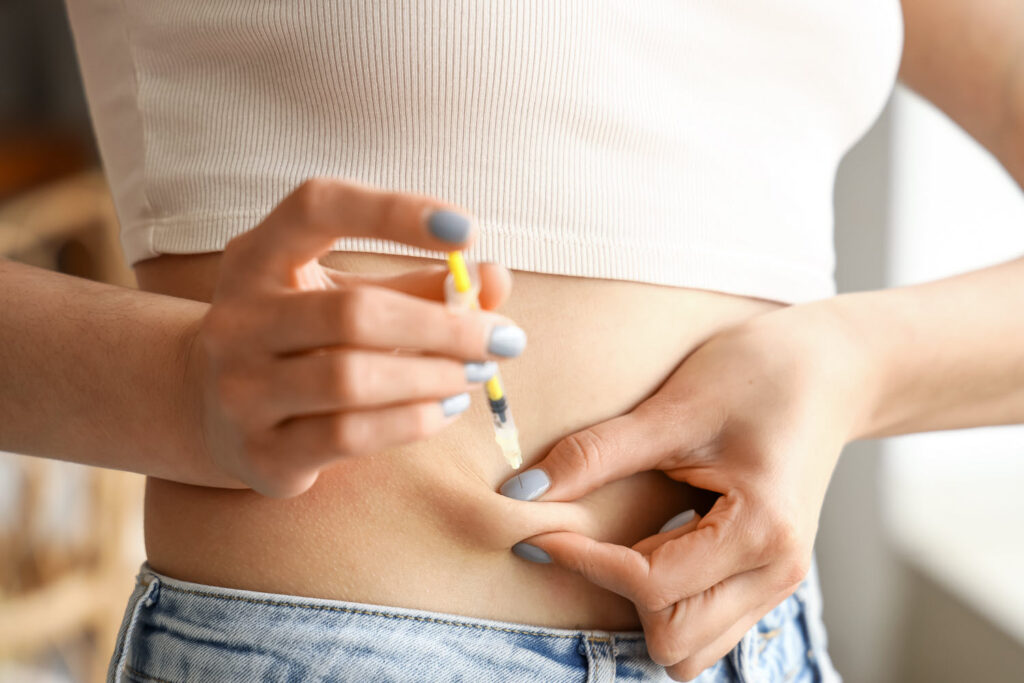
Using the Mounjaro Pen
The Mounjaro Pen is a single-dose pen designed for ease of use in administering tirzepatide injections. Proper preparation and technique are crucial for effective use.
Preparing for Injection
Before injecting Mounjaro, individuals should ensure they have the correct medication and dose. They must check the expiration date and inspect the mounjaro pens to ensure it is not damaged. It is important to keep the gray base cap on the pen until ready to inject.
Injection Technique
To administer the injection, one must first remove the base cap and attach the needle securely. After setting the dose, the user should press the button to inject the medication subcutaneously. Following the instructions for use is vital for proper injection technique.
Storing the Pen
Post-injection, the Mounjaro Pen should be stored at the correct temperature. Prior to first use, the pen is stored in the refrigerator. After the first use, it can be stored at room temperature, away from direct heat and light. Do not freeze the medication.
Injection Site Selection
Individuals can select from three injection sites: the thigh, abdomen, or upper arm. The site should be rotated each time to reduce the risk of tissue damage. One’s healthcare provider can assist in choosing the most suitable site for injection.
Post-Injection Care
After injection, it is important to safely dispose of the needle. The injection site should not be massaged, instead, one should monitor the area for any signs of irritation or adverse reactions. Proper post-injection care is essential for minimizing complications.
Dosage and Prescription
Mounjaro (tirzepatide) is a prescription medicine used for the treatment of type 2 diabetes in adults. Dosage and prescription for Mounjaro should be determined by a doctor, based on individual patient needs.
Dosage Guidelines
The starting dose for Mounjaro is typically 2.5 mg, injected subcutaneously once a week. Patients are generally advised to stay on this dose for a duration of four weeks. Following this period, the doctor may adjust the dose based on the patient’s response, increasing to 5.0 mg.
Week Dosage 1-4 2.5 mg 5+ 5.0 mg (subject to doctor’s recommendation)
Obtaining a Prescription
To obtain a prescription for Mounjaro, a patient must first see a doctor to determine if Mounjaro is an appropriate medication for their diabetes management. If prescribed, the patient can get the medication from a pharmacy.
It’s essential for patients to check with their insurance provider, as coverage for prescription medicines like Mounjaro can vary. Some patients may be eligible for savings programs which can be discussed with the doctor or pharmacist.
Health and Safety Information
Mounjaro pen, a medication for adults with type 2 diabetes, carries important health and safety information patients should be familiar with. This includes awareness of potential side effects, understanding contraindications, and taking special precautions based on individual health conditions.
Potential Side Effects
Patients may experience side effects, which could include nausea, vomiting, diarrhea, decreased appetite, and constipation. Some may experience swelling or an allergic reaction. It’s important to monitor for symptoms like a lump in the neck, hoarseness, trouble swallowing, or shortness of breath, as these could be signs of a rare but serious side effect, such as thyroid cancer.
Understanding Contraindications
Mounjaro is not recommended for individuals with a personal or family history of medullary thyroid carcinoma (MTC) or for those who have an endocrine system condition known as multiple endocrine neoplasia syndrome type 2 (MEN 2). It is also not for use in individuals with a history of pancreatitis or those with type 1 diabetes.
Special Precautions
Individuals should inform their healthcare provider of all the medicines they are currently taking to avoid potential drug interactions. If a dose is missed, follow the manufacturer’s guidance on when to take the next dose. Watch for symptoms of an allergic reaction, such as swelling or difficulty breathing, and seek medical attention immediately if they occur.
Lifestyle and Dietary Guidance
When managing one’s health with Mounjaro, it is important to focus on sustainable weight management and structured meal planning. These elements contribute significantly to the medication’s efficacy and overall well-being.

Weight Management
For individuals using Mounjaro, weight loss is often a primary goal. It’s important to adhere to a dietary regimen that supports this objective. Incorporating light and nutritious foods can promote a feeling of fullness without excessive calorie intake.
Pairing Mounjaro with a balanced diet that emphasizes vegetables, lean proteins, and whole grains can aid in achieving weight loss milestones.
Meal Planning
Structured meal planning is a cornerstone of dietary success, especially for those on Mounjaro. Planning meals ahead of time can prevent the consumption of unhealthy, high-calorie foods. By focusing on a diet rich in fiber and low in processed sugars and fats, one can better control blood sugar levels. Small, frequent meals are often more beneficial than large meals, as they can help with both weight management and maintaining steady glucose levels.
Additional Medication Information
When considering Mounjaro for treatment, healthcare professionals must be aware of its interactions with other medications and the implications of off-label use. Understanding these aspects is crucial for optimizing the effectiveness of the medicine and maintaining patient safety.
Drug Interactions
Mounjaro (tirzepatide), a medication used to improve blood sugar control in adults with type 2 diabetes, can have interactions with other medicines. It is particularly important to consider potential interactions with insulin or other drugs that affect blood sugar levels.
Co-administration with insulin or insulin secretagogues (such as sulfonylureas) may increase the risk of hypoglycemia. Therefore, a lower dose of insulin or the insulin secretagogue may be required to reduce the likelihood of low glucose levels.
Understanding Off-Label Use
While Mounjaro is approved for managing blood sugar in type 2 diabetes, any use outside of this indication is considered off-label. Healthcare providers might prescribe medications off-label when they believe it is medically appropriate for their patient; however, it is crucial that they are well-informed about the medication’s mechanisms, benefits, and potential risks. Since off-label use is not as extensively studied or approved by regulatory authorities, patients should be closely monitored for efficacy and safety.
Recognizing Serious Adverse Effects
Certain adverse effects associated with Mounjaro can be serious and require immediate medical attention. Cases of pancreatitis have been reported; symptoms to watch for include severe stomach pain that may radiate to the back, nausea, and vomiting.
Dehydration is another risk, potentially leading to kidney problems. Signs of dehydration include dizziness, weakness, and confusion. Patients with a history of stomach problems should be monitored as the medication might exacerbate gastroparesis, a condition characterized by delayed stomach emptying.
Allergic reactions can be severe, presenting with symptoms such as swelling of the face, lips, tongue, or throat, or difficulty breathing. There is a potential association between Mounjaro and thyroid tumors, including medullary thyroid carcinoma (MTC). Those with a personal or family history of MTC or Multiple Endocrine Neoplasia syndrome type 2 (MEN 2) should not use Mounjaro. If one observes a rash or other signs of a hypersensitivity reaction, seeking immediate medical care is critical.
Long-Term Safety
When it comes to the long-term safety of Mounjaro, more research is needed, as some risks may not be evident until after extended use. One such concern is the possibility of an increased risk of cancer, as indicated in animal studies. The FDA has approved Mounjaro; however, it’s crucial to be vigilant about any changes in health and to report any new symptoms to a healthcare provider.
Issues such as diabetic retinopathy may worsen with rapid improvement in blood sugar control. It is always recommended to monitor blood glucose levels to prevent episodes of low blood sugar, which can cause symptoms like irritability, sweating, and headaches. Regular check-ups can help in early detection and management of any serious long-term effects.
Frequently Asked Questions About Mounjaro Pen
1. What is The Typical Weight Loss Outcome When Using The 2.5 mg Dose of a GLP-1 Receptor Agonist?
The 2.5 mg dose of a GLP-1 receptor agonist, such as Mounjaro, is typically for treatment initiation. Typical weight loss outcomes are visible after a few weeks of use.
3. What Are The Available Doses for This Weekly Injectable Medication?
Mounjaro is available in multiple doses, starting at 2.5 mg and possibly being increased to 5 mg, with subsequent dose adjustments made based on the patient’s glycemic response and tolerability. These adjustments are to be made over time with medical oversight.
4. What is The Recommended Starting Dose of This Medication for Non-Diabetic Patients Focusing on Weight Loss?
For non-diabetic patients focusing on weight loss, the recommended starting dose of this medication is typically the lowest available dose, which is usually 2.5 mg once weekly. The provider may increase the dose as needed, taking into account the individual’s response and tolerability.
5. At What Point Do Patients Reach a Maintenance Dose With This Medication, and What Factors Influence This?
Patients may reach a maintenance dose with this medication generally after several weeks to months, depending on their response and the presence of any side effects. Factors such as individual efficacy, tolerability, and specific weight loss goals will guide the dose adjustment process.
6. Is It Possible to Obtain This Medication for a Reduced Cost, and What Steps Need to Be Taken?
It is possible to obtain this medication for a reduced cost. Patients may need to check if they qualify for savings cards or patient assistance programs. They should consult their healthcare provider or look into available Savings Cards.

Conclusion and Summary of Mounjaro Pen: Guide to Using for Weight Loss
Incorporating Mounjaro into your diabetes management plan requires an informed approach, understanding not just the benefits but also the intricacies of dosage, administration, and potential side effects.
By staying informed about your scheduled day for injection, how to manage a missed dose, and what to do in the event of possible side effects, you’ll be better equipped to handle your treatment.
Always maintain open communication with your healthcare provider, discuss any concerns about thyroid c cell tumors or other serious risks, and ensure you have access to all the necessary resources to manage your condition effectively. Your path with Mounjaro can lead to better health outcomes when navigated with care and knowledge.
Please note that this article is intended for informational purposes only and should not be construed as medical advice. Before making any changes to your treatments, please consult with your healthcare provider to discuss the appropriateness and safety of such changes.
Ready For Your First-Class Cosmetic Experience in Orange County (OC) California (CA)?
Are you located in one of these Orange County (OC) / Southern California cities?
Aliso Viejo, Anaheim, Brea, Buena Park, Costa Mesa, Coto de Caza, Cypress, Dana Point, Fountain Valley, Fullerton, Garden Grove, Huntington Beach, Irvine, La Habra, La Palma, Laguna Beach, Laguna Hills, Laguna Niguel, Laguna Woods, Ladera Ranch, Lake Forest, Los Alamitos, Mission Viejo, Newport Beach, Orange, Placentia, Rancho Santa Margarita, San Clemente, San Juan Capistrano, Santa Ana, Seal Beach, Stanton, Tustin, Villa Park, Westminster, or Yorba Linda?
Plastic Surgeon Dr. Brandon Richland, MD and our Cosmetic Aesthetics Team are ready to help you look and feel your absolute best.
Elevate your confidence and self esteem levels to unfathomable new heights!
Schedule your in-person consultation in our modern and luxurious offices in either Fountain Valley, CA (Main HQ) or our Newport Beach, CA office.
Do you live outside of Southern California or short on time? For your convenience, Virtual Consultations are also available.
Our warm and engaging Team of carefully selected Aesthetics Professionals will make you feel calm, cool, collected, and right at home throughout your entire consultation and surgery process.
Schedule Your Aesthetics Consultation here, or call us directly at 949-867-4496 today.
About the Author

Dr. Brandon Richland, MD is a respected Board Certified Licensed Plastic Surgeon in Orange County / Southern California specializing in cosmetic and reconstructive surgeries.
Driven by his passion for medicine, Dr. Richland obtained his Doctor of Medicine (M.D.) degree from the prestigious program at Saint Louis University (SLU) School of Medicine in 2013. His exceptional skills were recognized when he received the McGraw Hill / Lange Medical Student Academic Achievement Award, and graduated top of his class with Honors. For his undergraduate degree, he attended University of California, Los Angeles (UCLA) and graduated with Honors in 2009.
To further enhance his surgical expertise, Dr. Richland completed his Residency in Plastic Surgery at the University of California, Irvine (UCI) from 2013 to 2019 earning the Academic Achievement Award twice during this period. A total of 14 years in dedicated schooling and medical residency.
Dr. Richland is actively involved with healthcare and medical societies, as a Diplomate of the American Board of Plastic Surgery, a member of the American Society of Plastic Surgeons, American Society of Aesthetic Plastic Surgeons, and the California Society of Plastic Surgeons.
Contact Dr. Richland today by visiting RichlandMD.com, scheduling a cosmetic consultation, or by calling 949-867-4496 directly.










